When thinking about what manufacturing looks like, most people envision sparks flying, metal clanking, and fires blazing. This isn’t too far off the mark since metals were used to bond joints together to build larger, sturdier, and more stable structures before the first Industrial Revolution, making these processes the cornerstone of manufacturing. We’re on the cusp of what many call the Fourth Industrial Revolution (4IR), and creating strong joints between metals is still at the forefront of production for many industries.
Brazing is a relatively simple and effective way to form a powerful and resilient bond, especially if you have dissimilar metals to assemble. Brazing utilizes a filler metal that is heated to its melting point and spread over two metal pieces at a joint. The filler metal (brazing alloy) has a lower melting point than the base metals being joined, so the molten alloy can flow over the adjoining metals to create a seamless bond when the metal hardens again.
Among the many ways to create joints between metal materials, brazing is one of the most widely used in the production of heat exchangers. Heat exchangers are typically built by bonding several metal plates and/or metal tubing together to decrease the temperature of the liquid flowing through them. They are used to cool large electronics assemblies at data centers, medical equipment used for CT and CAT scans, automotive engine cooling, and HVAC cooling. Brazing is also a common way that metallurgists make fine jewelry.
In order for brazing to work properly, the base metals need to be clean enough for the filler metal to flow freely over them without any interference. Achieving this cleanliness means the metals undergo a preparation process before the brazing can commence.
But what is happening to the surface of the metal as it gets cleaned and prepared, and how can we know for sure that once we start melting our brazing alloy, we’ve set it up for success?
Rethink your adhesion manufacturing processes with Surface Intelligence.
How Does Metal Brazing Work?
Brazing utilizes the melting of an additional filler metal, other than the two being joined, to create the joint. With welding, the two base metals and the filler metal are melted and formed together. With brazing, like soldering, a brazing alloy (usually a combination of copper, silver, aluminum, phosphorus, zinc, or nickel) in the form of a rod, powder, or wire is heated up to above 840° F or just above the melting point for the particular metal alloy. It is then applied to the gap between the base metals and flows over them using what is called capillary action. The base metals are often copper, aluminum, brass, bronze, or stainless steel, and sometimes ceramics.
Brazing is often confused with welding, but it has a couple of key differences. For starters, the base materials never melt, so the temperatures are always lower. Concentrating the heat on the melting point of the brazing alloy makes it orders of magnitude, making it easier to braze dissimilar metals. With welding, you need to be cognizant of the variance in melting points and use sophisticated welding techniques to ensure each metal is sufficiently melted to make the joint.
To learn more about how to ensure your brazing, welding, soldering, cleaning or adhesion process are optimized to create the most reliable products, download our eBook: The Manufacturer’s Roadmap to Eliminate Adhesion Issues in Production
Another characteristic distinguishing brazing from welding is that brazed joints are arguably much more attractive than welded ones. If done properly, the brazing alloy will melt into a smooth and tidy joint, as opposed to the inconsistent look of a thick, irregular bead characteristic of a welded joint. Brazed joints require little to no finishing operations to make them showroom-ready.
The Magic of Science and Capillary Action
Sometimes, brazed joints are referred to as “self-made.” It’s not exactly accurate, but it gets at a key aspect of brazing that makes it a relatively easy metallurgic bonding technique.
When the brazing alloy heats beyond its melting point, and the base metals have been sufficiently cleaned and prepared, the alloy will flow in the gap between the metals and expand on its own, seeming to create the joint out of the sheer will. This phenomenon is not actual magic but a scientifically founded occurrence that works because of our old friends, Physics and Chemistry.
Capillary action or capillary flow is the act of a liquid “pulling” itself over a surface. The ability for capillary flow to fully work and for the liquid to pull itself across the surface sufficiently relies on two things: the gap between the base metals and the cleanliness of those metals.
The gap between the base metals must be wide enough to let the molten alloy through, but the wider the gap gets, the less capillary action takes place. This decrease is because capillary action is determined by the molecular attraction between molecules on the surface of the brazing alloy and the molecules on the surface of base metals.
To illustrate this, let’s look at a cup of water with a straw placed in the water. When you place a straw in the water, you’ll notice that the water moves up the straw and is higher than the water level in the cup. There are attractive forces acting on both the molecules on the surface of the inside of the straw and the molecules in the water. The water molecules are attracted to the molecules on the straw’s surface, and they are also attracted to themselves. The attractive forces between the straw and the water are stronger at a closer range, so if the diameter of the straw gets wider, the water would slip down closer to the level of the water in the cup.
These attractive forces exerted by the molecules on the straw and the water molecules are called surface energy and surface tension, respectively. To make the water more attracted to the straw and increase the capillary action, the surface energy of the straw needs to be increased to surpass the energy or surface tension of the water. Increasing the surface energy requires altering the chemistry of the straw's surface.
To conduct a surface energy experiment at home, try taking a reusable aluminum straw, a reusable silicone straw, and a paper straw and placing them in water to see how the water reacts to these different materials. Materials have different innate surface energies (e.g., on the whole, metals have much more reactive surfaces than polymers, and some polymers, like PTFE, basically don’t react with anything), and the water should stand at different heights in the straws depending on how attracted the molecules are to the surfaces of the straws as opposed to the water.
So, how does this illustration relate to brazing metals? Think of the base metals as the straw in the cup of water. To make the molecules on the surface of the molten alloy sufficiently attracted to the molecules on the surface of the base metals, the base metals need to have high surface energy.
To increase the surface energy of the metals, you need to clean their surfaces sufficiently. In the assembly process of many of the heat exchangers mentioned above, the metals used will be fresh and new, which can give manufacturers a false impression of safety. Since we are talking about a chemical reaction pulling the liquified alloy over the other metals, we need to be sure that our metals are chemically clean. Oils, grease, and other residues from aerosols in the manufacturing environment can make their way to the base metals anytime. These contaminants will destroy capillary action because they decrease surface energy. After all, the molecules inherent in these residues are not attractive to the alloy. Most of these oils and greases are lubricants designed to ensure things don’t stick together. If that’s on your base metal surface, capillary action just isn’t going to happen.
Revolutionize Your Manufacturing with Surface Quality Inspection Technology.
Oils, cutting fluids, and other incidental residues can be cleaned with a solvent wipe, vapor degreaser, or aqueous washer. Then, the metals should be further cleaned of debris with a mild abrasive like an emery cloth. If you’re doing a brazing repair in the field and the metals are heavily soiled or rusted, you might need a grinder and heavier abrasion, but be aware that this could drive oils into the surface and make them harder to clean off after the fact.
It’s also crucial to be certain of the cleanliness of your incoming metal parts so you know what you’re working with. Not all contaminants are treated equally, and you might need to take a different approach depending on what’s on the surface of your metals. When manufacturers experience joint failures, even though they clean their surfaces for all the contaminants they know about, it could indicate that there are unexpected contaminants making their way onto the surfaces. To determine what residues are present at a molecular level, they may need to enlist the help of a Surface and Materials Lab that can use techniques like x-ray spectroscopy and infrared spectroscopy to gain insight into what’s on the surface and how to best remediate it if it is indeed interfering with adhesion.
How to Properly Use Flux for Brazing
Continuing the chemistry discussion, it must be said that oxidation in the form of rusting is a major surface energy inhibitor. When the base metals have oxidized and formed a layer of rust, even the slightest bit, it can impair capillary flow and will also create a weak bond. When there is even a thin layer of rust on the surface, the joint will break within the rust layer when stress is put on the joint because the adhesion between the rust and the metals will not hold. This is why a substance called flux is often used to mitigate the oxidation of the metals, which is accelerated by heating.
Flux is typically a chemical compound in the form of a paste that is brushed on the surface in the case of low-volume brazing. Dipping in the flux may be more suitable in high-rate manufacturing, or depositing the paste with an automated applicator gun.
The flux must completely cover the joint and be applied immediately before introducing heat. Some fluxes will become transparent when the proper temperature is reached. The flux will dissolve and absorb any oxides on the surface of the base metals, making it safe to begin applying the brazing alloy.
How to Make Sure Your Base Metals are Clean Enough for Brazing
In Brighton Science's experience with manufacturers who use brazing to create joints in their production process, knowing if their metals are clean enough before brazing is their greatest challenge. If there is a failure in the joint after the final assembly is complete, there may not be an opportunity to repair it. The failed joint is likely in a place within the structure that cannot be easily reached. Many of the heat exchangers mentioned earlier are part of large assemblies that cannot be removed from the building they’re in and are extremely expensive to repair.
Consistent and flawless performance is crucial in many products that are built using brazed metals.
The best way to predict if capillary action will work its magic is to do a test that mimics this flow beforehand. Typically, manufacturers will do a stress and strength test after the brazing, but it is much more cost-effective and time-efficient to preemptively calculate whether or not the cleaning process was carried out properly.
Using a water contact angle is a testing method that measures the behavior of a drop of water on a surface to gauge the surface energy of the material in question. This is the perfect test for brazing because the way the water droplet wets out or flows on the base metals' surface clearly indicates how the brazing alloy will react to that same surface.
Below is a graph created by the scientists at Brighton Science for a customer who was trying to replace a subjective and qualitative test of wettability with a water contact angle measurement. The customer had brazing foils that didn't always flow on the metal surfaces as they needed them. They wanted to use contact angle measurements to predict if their brazing foils would wet out properly and create strong joints.
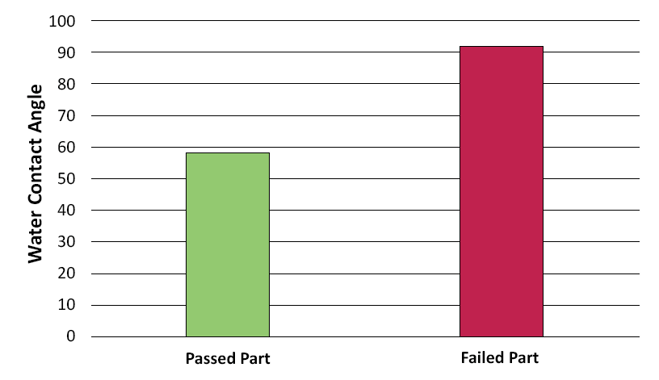
The graph shows that the samples that did not wet out properly had higher contact angle measurements (which means they had lower surface energy) than the ones that did wet out. This means that the customer could rely on the results of contact angle tests to predict the behavior of their materials.
Some of the greatest advantages of these tests are that they are non-destructive and can be done directly on actual parts on the production line. The equipment used to take contact angle measurements can be automated, so it fits perfectly with any high-rate manufacturing process. There are also portable devices designed for field inspections when repairs to brazed joints are necessary.
Download our roadmap to learn more about optimizing your brazing, welding, soldering, cleaning, or adhesion process to create the most reliable products. This eBook offers a wealth of insight into manufacturing processes and the unforeseen obstacles that engineers face but do not have to face alone. Download the free eBook today: The Future of Manufacturing: A Guide to Intelligent Adhesive Bonding Technologies & Methodologies.