A major packaging company was having intermittent problems heat sealing a film used to package paper products. Dyne pens, used to measure surface energy, showed no differences between a “good” film, one that heat sealed appropriately, and a “bad” film, one that did seal correctly.
Brighton Science found that dyne pens cut through a contaminant found on the bad films, leaving the packaging company with a false sense of security and inevitably scrambling to understand why they were disappointing their customer. Water contact angle measurements were able to detect the contamination and provided a better gauge to ensure films were ready for heat sealing.
When Brighton Science analyzed the films, water contact angle was the first tool we used to baseline the differences between films. We found higher water contact angles on the good film compared with lower contact angles on the bad film (Figure 1). This was unusual for Brighton Science, as usually a higher contact angle indicates a less-active surface that will not bond or seal correctly, but in this case the lower contact angle was not bonding correctly.
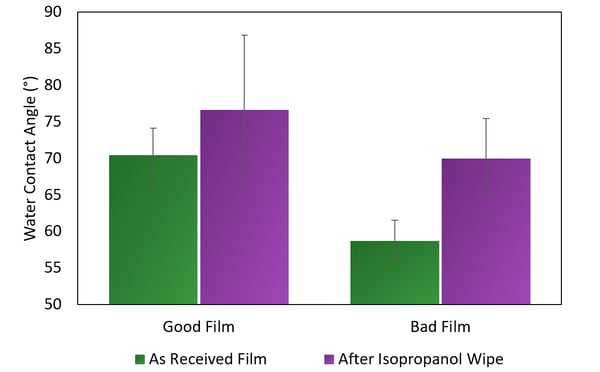
Figure 1. Water contact angle measurements on known good and known bad films before and after a wipe with isopropanol.
The films were then analyzed using infrared spectroscopy. Brighton Science was able to remove different substances from the films and identified that the bad film contained an amide group that the good film did not have (Figure 2). Amide groups are generally hydrophilic, while the polymer film is hydrophobic, so it answered the question of why the bad film had a lower contact angle. But now there was a new question: where was the amide coming from?
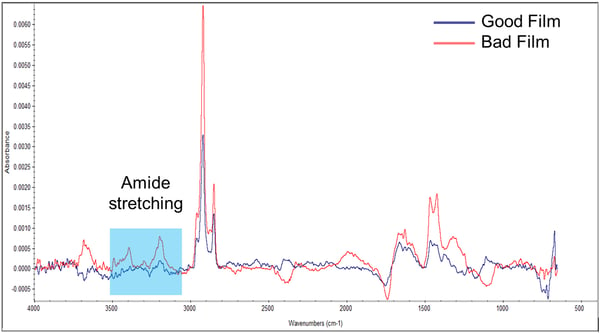
Figure 2. Infrared spectra of known good and known bad films.
The answer was contained in the film additives. This blown film requires a lubricant, also called slip, in the formulation to reduce friction on the film as it passes through rollers and corona treatment during the cooling process. The slip molecule, a fatty acid amide, contains an amide group, which was identified through chemical analysis. This was present on the bad films, not the good films. These processing aids are known to reduce friction but can cause challenges for adhesion and heat sealing when present in excess.
There are several reasons why excess slip could appear on the surface of a film. There may have been too much slip added to the film formulation of this packaging. There could have been leftover slip on the rollers from a previous batch of a different kind of film. A hot day can cause more slip to migrate to the surface. No matter the cause of the excess slip, the film manufacturer now has a way to measure and monitor it because it can be found using a water contact angle.
Rethink your adhesion manufacturing processes with Surface Intelligence.
As a final question, the film manufacturer wondered why dyne pens did not pick up on the difference in surface energy between the good and bad films. Slip is soluble in alcohol and many other solvents, including those found in dyne pens. In this case, the dyne pen ink could not indicate the presence of slip because it dissolved the ink. However, slip, while somewhat hydrophilic, is not very soluble in water, so water could react to its presence without dissolving it (Figure 1).
The film manufacturer invested in a Surface Analyst, a method of measuring contact angle, and now has a means of monitoring excess slip in their process. This tool can gauge different surfaces, so it can be used on the rollers to discover leftover slip residue between batches, change corona treatment levels to change slip migration, determine when a hot day of production is too hot for slip migration, or investigate any other potential causes for excess slip.
For more information about how to discover the source of contamination, especially when it isn't immediately obvious, download our eBook with a step-by-step guide for starting your own investigation. Download the eBook today: Checklist: Adhesion Failure Root-Cause Analysis for Manufacturers.


