By Dr. Giles Dillingham
Why One Contact Angle Fluid is All You Need to Control Your Process
Contents
Abstract
Polar interactions between a properly prepared surface and an ink, adhesive, or coating are strong and are responsible for most of the required adhesion of inks, paints, and adhesives. These interactions are greatly affected by cleaning processes and surface treatment. Non-polar (or dispersive) interactions are relatively weak, are insensitive to cleaning and surface treatment, and their measurement requires the use of hazardous fluids.
As a result, the best metrics for measuring and controlling the surface properties important for adhesion are the ones that are most sensitive to the polar characteristics of a surface. A single water contact angle measurement provides this sensitivity without the need for multiple probe fluids.
Rethink your adhesion manufacturing processes with Surface Intelligence.
Surface Energy and the Attractions Between Substances
Solids and liquids exist because all atoms and molecules attract each other. Even gases with low intermolecular attraction (think helium or nitrogen) become liquids if they are cooled enough. The same intermolecular attractive forces that hold a substance together will attract other substances, and these forces are what causes the molecules in a paint or an adhesive to stick to the molecules that comprise a substrate.
The force of attraction of one molecule for another depends on the structure of the molecule: some structures are strongly attractive while some are only weakly attractive. Treating a surface through cleaning or oxidizing with a flame, corona, or plasma can cause it to attract a paint or adhesive more strongly because these treatments replace weakly attractive molecules on the surface with more strongly attractive ones.
One way to characterize the attractiveness of a surface towards other substances is by a property called surface energy: a high energy surface is generally attractive to other substances; a low energy surface is not.
Examples of high energy surfaces are clean metals or glasses: Paints and adhesives stick to them strongly. Low energy surfaces are exemplified by substances like polyethylene or polytetrafluoroethylene (Teflon™): paints, adhesives, foods, and most other things are not attracted to these surfaces and do not stick.
All liquids and solids have the property of surface energy. The surface energy of a liquid is usually referred to as its surface tension, but surface tension and surface energy are the same thing. While Scientists usually speak of surface energy as energy per unit area (millijoules per m2); Manufacturing engineers usually use units of surface tension (force per unit length, or dyne/cm). Both units are dimensionally equivalent.
Controlling Surface Properties
Controlling a cleaning process or surface treatment process requires a way to measure the surface characteristics that are affected by these processes. There are several ways to do this. We can analyze the molecular composition and structure directly through the use of analytical instruments like an X-ray Photoelectron Spectrometer (XPS) or an infrared spectrometer (FTIR).
X-ray Photoelectron Spectrometer (XPS)
These techniques are expensive and not usually very practical for use in process control in a manufacturing facility. Considering that the ability of the surface to attract or repel another substance is the fundamental property of interest, a direct measurement of this ability is in many ways the ideal control metric.
This concept is the basis of wetting measurements using dyne inks or contact angle-based methods: both are techniques for probing how strongly a surface attracts a liquid. Both methods are commonly used in industry, although they differ greatly in their accuracy and precision.
Dyne inks are the most frequently encountered method. Dyne inks consist of a series of liquids having a range of surface tensions (surface energies) created by mixing two or more mutually soluble liquids of different surface tensions in different proportions. Commercially available mixtures include ethanol and water or formamide and 2-ethoxyethanol. A dye is added to the mixtures to improve visibility on the surface. In use, a thin film of the liquid is spread on the surface to be tested; if the liquid film breaks up into droplets in less than two seconds, the test is repeated with a mixture of lower surface tension.
The surface tension of the liquid mixture that will stay spread in a continuous film on the surface for at least two seconds is defined as being equal to the ‘wetting tension’ of the surface. This ‘wetting tension’ number is used as an approximation of the surface energy of the surface under evaluation but suffers from low accuracy and precision as well as poor reproducibility. Furthermore, because dyne inks are solvents, they clean the surface of contaminants during measurement and therefore frequently do not detect them.
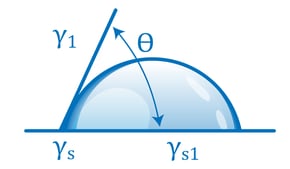 Contact angle-based methods are much more quantitative and much less subjective than dyne ink methods. They involve measuring the way a drop of liquid placed on the surface spreads, usually by measuring the angle formed by the perimeter of the drop and the surface. A liquid drop that spreads a lot has a small contact angle; this means it is attracted to the surface more strongly than to itself. A drop that spreads little (‘beads up’) has a large contact angle: it is being attracted more strongly to itself than to the surface. The contact angle of the liquid on the surface changes with the state of the surface in a very predictable and reproducible way.
Contact angle-based methods are much more quantitative and much less subjective than dyne ink methods. They involve measuring the way a drop of liquid placed on the surface spreads, usually by measuring the angle formed by the perimeter of the drop and the surface. A liquid drop that spreads a lot has a small contact angle; this means it is attracted to the surface more strongly than to itself. A drop that spreads little (‘beads up’) has a large contact angle: it is being attracted more strongly to itself than to the surface. The contact angle of the liquid on the surface changes with the state of the surface in a very predictable and reproducible way.
For example, clean metals, ceramics, and glasses establish low contact angles. Contamination of these surfaces causes the contact angle to increase: the liquid is not attracted strongly to the contaminant. Untreated polymers have high contact angles; oxidizing the surface of a polymer by corona, flame, or plasma causes the contact angles to decrease: they attract the liquid more strongly.
The contact angles change up or down in a smoothly predictable way with the level of contamination or the treatment level. This is why they provide an excellent metric for process control.
Why Substances Attract Each Other
Materials, substances, and the objects they make up stick to each other for a simple reason: the molecules that they are made of attract each other. The attractions between molecules are ultimately based on the attractions between the negative and positive charges of the electrons and protons that make up the molecules.
A molecule consists of atoms that are bound together to form a certain shape. If we were small enough to hold a molecule in our hand and look at it like we would any small object, we would see a cloud of negative charge (the electrons) defining the shape on the outside with the nuclei (containing the positive protons) hidden on the inside of this electron cloud. The electron cloud is always in random, fluid motion. While it has a well-defined average shape, because of its random motion it will be concentrated over one part of the molecule for one instant and concentrated over another part in the next instant.
When the electrons are momentarily concentrated over one part of the molecule or another, that part of the molecule will have an excess negative charge, leaving the rest of the molecule with a slight (and compensating) positive charge. This charge distribution is called a temporary dipole; they exist in all atoms and molecules that are above absolute zero (at absolute zero, the electron motion stops, so no temporary dipoles can form).
Because positive and negative charges attract each other, the temporary dipoles of atoms and molecules attract each other. This hold substances together. The attractions between temporary dipoles is why, for example, vegetable oil doesn’t evaporate, but exists as a liquid in an open container. These forces are called dispersive forces, because they originate in the random dispersion of the electron cloud around an atom or molecule. Because all atoms and molecules have randomly fluctuating electron clouds, these dispersion forces exist between all atoms and molecules, regardless of the structure or composition. Big, complex molecules with large electron clouds will have stronger dispersion forces, while smaller, simpler molecules will have weaker dispersion forces, but in all cases they are relatively weak. We have little or no control over the magnitude of dispersion forces between molecules.
Surface treatments via plasma, corona, or flame treatment will change the dispersion component very little (or not at all) for simple polymers like polyethylene and polypropylene. For polymers with more complex structures, like epoxies or polyesters, surface treatments usually results in some level of decrease in the dispersive attraction between the molecules, probably because the surface treatment reduces the size and complexity of the molecular structure at the surface.

Atmospheric Plasma Treatment
There is another, much stronger, attraction that can exist between molecules that depends much more on the specific structure and composition of the molecules. Nitrogen and oxygen are elements that attract electrons more strongly than carbon. If oxygen and nitrogen are present in a molecule that is otherwise mostly carbon and hydrogen, the electron cloud tends to concentrate in density around these elements. This makes the part of the molecule containing oxygen or nitrogen more negative, leaving the rest of the molecule slightly positive. These dipoles are permanent, not temporary. These polar forces between permanent dipoles attract each other perhaps 100X more strongly than the temporary dipoles responsible for dispersive forces.
Some polymers, such as polyesters, polycarbonate, or acrylics, have these permanent dipoles as part of their structure. This is why they can frequently adhere well to adhesives, paints and inks with no additional treatment. For other polymers, like polypropylene or polyethylene, oxygen or nitrogen can be grafted to the polymer surface by a plasma, corona, or flame treatment, providing greatly improved adhesion.
Because polar interactions are so strong, the polar character of a surface is very important for adhesion and can be controlled by surface treatments. The dispersive part of the surface energy is small, relatively unimportant for adhesion, and is much more difficult to control. As an example, the accompanying Figure shows how the polar and dispersive components of surface energy of polyethylene change as the total surface energy is increased by plasma treatment. It’s clear in this case that the polar component is affected most by plasma treatment.
.png?width=600&name=plasma-treated-polyethylene-graph%20(6.8.20).png)
In other cases, as shown in the next Figure, the total surface energy remains relatively constant because the increase in the polar component is largely negated by a decrease in the dispersive component. These surfaces still show excellent improvement in adhesion to adhesives, inks, and coatings, however, because the polar interactions with the coating or adhesive are so much stronger than the dispersive interactions.
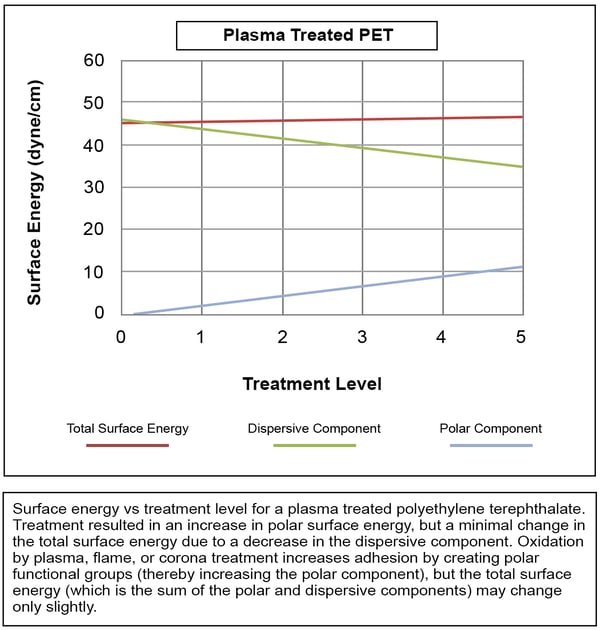
Measuring Surface Treatment via Contact Angle Methods
The total attractive force between substances is the sum of the two basic types of attractive forces between substances: the strong polar forces that exist between molecules that have permanent dipoles, and the weak non-polar (dispersive) forces that exist between all molecules. To determine the contribution of each of these two different forces to the surface energy of a substance, we must make contact angle measurements with at least two different liquids that have known (and different) polar and dispersive contributions to their surface tension.
This is the basis for most commercial methods, and the results agree well with other, absolute methods for measuring surface energies. It’s important to note that although many commercial software packages use contact angles measured from two fluids to calculate surface energies, the error can be quite large. The precision of the surface energies calculated from contact angle measurements is greatly improved if more than two fluids are used.
Revolutionize Your Manufacturing with Surface Quality Inspection Technology.
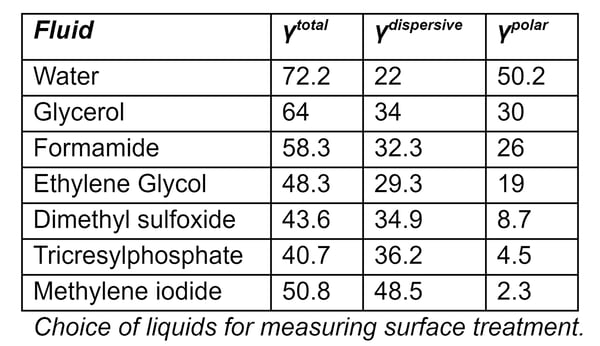
Choice of Liquids for Measuring Surface Treatment
The accompanying Table shows liquids that are commonly used for measuring surface energies, along with the dispersive and polar components of their surface tensions. The two most common liquids used for measuring total surface energy are water (a highly polar liquid) and diiodomethane (which is a highly dispersive liquid with very little polarity).
Because the polar part of a liquid only interacts with the polar part of a surface, and the dispersive part of a liquid only interacts with the dispersive part of a surface, polar liquids (e.g. water) have contact angles that are respond strongly to the polar component of surface energy. Conversely, liquids whose surface tension is mostly dispersive (methylene iodide, for example) have contact angles that do not change very much as the polar component of the surface energy changes. 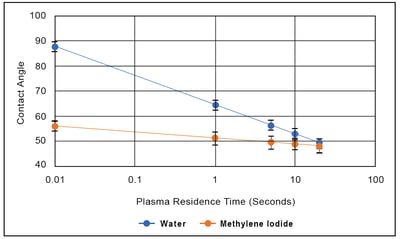
The accompanying Figure shows the effect of plasma exposure time on the contact angles for water and methylene iodide on polypropylene. The contact angle for methylene iodide changes a small amount with plasma treatment (the Table shows that is has a small but non zero polar component to its surface tension), but the change in contact angle it not much greater than the standard deviation of the measurement: this is a poor metric for process control. (Furthermore, the toxicity of methylene iodide make it a poor choice for deploying in a manufacturing environment). On the other hand, this Figure shows that the contact angle of water is very sensitive to treatment level: the treatment is primarily changing the polar character of the surface, and water is an extremely polar liquid. There is a one-to-one correspondence between water contact angle, contamination level, and surface treatment level that make it in many ways an ideal metric for controlling surface treatment.
Conclusions
All substances attract all other substances through at least two basic mechanisms: the weak dispersive forces that exist between all atoms and molecules regardless of structure, and the strong polar forces that depend on the details of the molecular composition and structure. The dispersive component of intermolecular interactions contributes little to adhesion in most cases and changes little with treatment. Because a decrease in the dispersive component of surface energy can mask changes in the important polar component of surface energy, using the total surface energy as a metric to control surface treatments can be misleading.
The polar component of intermolecular attractions is responsible for adhesion enhancement of surface treatments such as corona, flame, and plasma treatments, and can be controlled over a wide range; a simple water contact angle measurement is frequently fills all the requirements for a sensitive process control metric.
Download Your Copy of This Paper
Download your personal copy of this paper by completing the form to the right.

