The attractive force of the molecules present at the surface of a liquid towards each other is called the surface tension of that liquid.
It may seem like a little thing (and in terms of mass, it doesn’t really get much smaller than the top few molecular layers that make up the surface of a liquid or solid), but the attractive forces that pull these molecules toward each other, are radically powerful in their impact on manufacturing processes. These forces are the unseen glue that holds modern manufacturing together, if you’ll pardon the somewhat literal pun.
Many things must be managed and controlled for a manufacturing process to output reliable, high-performing products. Many of these things are highly controlled and have a long history of being studied, measured, and contemplated. There are others, however, that are completely missed due to not realizing their importance and not having the tools available to measure them.
Chemical laws govern how reliably and strongly a liquid, like an adhesive, ink, or coating, will adhere to a solid. Measuring surface tension is part of the equation to predict how strong and reliable that adhesion will be. It’s not enough to merely know that scientific principles are a part of manufacturing processes. If you do not have quantitative evaluations of invisible forces, such as surface tension or surface energy, then you are leaving your process vulnerable to producing substandard products.
It’s true what they say: you can’t manage what you don’t measure.
Rethink your adhesion manufacturing processes with Surface Intelligence.
The performance of a bond or coating, such as paint on a commercial airliner, the glue holding a glass touchscreen on a phone, or the polymer coating on the end of an electrical cord protecting a life-saving ventilator from malfunctioning, relies on the chemical properties of the top few molecular layers at the surface of liquids and solids.
Measuring the attractive force between these molecules offers more than just one more data point in a long list of data points; it’s a prescient peek into the future of your products. The predictive analytics from these measurements of molecular behavior is an insurance policy against warranty claims, returns, recalls, and unhappy customers.
Why is Surface Tension Important?
Surface tension is important because our world is bound together by the forces that attract molecules to each other. These forces govern how substances interact with other substances, and the most basic interaction between substances is through their surfaces. Surface tension impacts how they behave in this interaction. The attractive force between the molecules on these surfaces can be understood and controlled by measuring the surface tension or surface energy. Because if we are going to understand and control manufacturing processes, we need to control how surfaces interact with each other.
To learn more about how to use surface energy measurements to create more reliable products, download our free eBook: Predictable Adhesion in Manufacturing Through Process Verification
Surface tension is a measurable value that tells us how strong the attraction between molecules on the surface of a liquid is. The chemical characteristics that determine how high the surface tension of a substance has implications on other characteristics of that substance. For instance, high surface tension substances tend to have high boiling points: they have to be heated up a lot in order to overcome the intermolecular attractions to break them apart and form vapor or steam.
By knowing the surface tension of a substance, you can gain a deeper understanding, as well as control, of how that substance will behave in an adhesion process.
Surface Tension and Temperature
Many factors affect the surface tension of a substance. In fact, anything that affects the attraction between molecules affects surface tension.
Temperature is one of many variables that can cause surface tension to vary, but compared to other variables, it has a relatively tiny effect. The molecular structure or composition of a substance is of far greater concern than temperature. Surface tension is 99%+ dependent upon the chemical composition of the molecules that are present in the top 1-5 molecular layers of a substance’s surface. Comparatively speaking, the temperature has a negligible effect on surface tension.
The temperature has a much more substantial impact on the viscosity of liquids than it does the surface tension. Viscosity is a measure of the resistance to flow or, more precisely, how hard it is to move liquid molecules past each other. Viscosity is not a measure of how strongly attracted those molecules are to each other or to other molecules.
Factors Affecting Surface Tension
So, let’s talk more about the molecular composition of surfaces since they have such an outsized effect on surface tension.
A liquid can be a single-component substance (i.e., it’s a homogenous group of like molecules), or it could be a multi-component substance (i.e., consisting of a blend of various substances and; therefore, the molecules are not all the same). And so, if you’re trying to determine the surface tension of a single component liquid, it only depends on the molecular structure of that liquid since it’s made up of all the same stuff. If you’re dealing with a multi-component liquid, the surface tension depends on the structure and amount of the individual components that make up the liquid.
In manufacturing processes, it is common to be using multi-component substances like inks, paints, or melted polymer blends.
It’s critical to note that surface tension is not necessarily the sum of the surface tensions of each component. For instance, if you have 75% of component A and 25% of B in your liquid, you do not simply use the weighted average of the surface tension of both substances to get the surface tension of the whole liquid. It is less a matter of the quantity of components and more a matter of chemical characteristics. Different molecules have differing chemical attributes that affect how attractive they are to each other and molecules of other substances.
Surface tension is really determined by the way the molecules arrange themselves on the surface of the liquid. And it’s their chemical properties that influence how they arrange themselves. Suppose you have components that interact with each other strongly. In that case, the molecules can affect each other in a manner that changes the relative amounts and orientations of the molecules at the surface. The result is a change in the overall surface tension that can be hard to predict.
Dynamic Surface Tension Measurement
Surface tension is a static property; however, it can be changed and manipulated by altering the chemical properties of the molecules at the surface of a liquid. For example, you can add some surfactant to an ink to change the surface tension and make it spread on the surface of a solid paper.
Dynamic surface tension refers to the measurement and understanding of how the changes you’re implementing to the overall composition of a liquid affects the surface tension of that liquid. For example, immediately after adding a small amount (say. 0.1%) of a surfactant to water, the composition of the surface of the water will only have changed 0.1%, and we will be unlikely to be able to measure a change in surface tension. However, a second or two later, when the surfactant molecules have had an opportunity to diffuse to the surface, the surface concentration of surfactant could now be close to 100%, and we will measure a large drop in surface tension. If we then agitate the water, we can disrupt the surface and reduce the concentration of surfactant molecules there, raising the surface tension back up towards the initial value. Using surface tension measurements to control manufacturing in this situation requires knowledge of its response to mixing: measurements have to be made dynamically, meaning while paying attention to time and mixing dependence. This is very important in manufacturing products that include surface-active agents like surfactants and wetting agents.
Surface Tension Meter
In order to measure surface tension at various points in the life of a liquid, a surface tension meter is used to directly measure the surface tension of a liquid. It’s important to note that the surface tension of a liquid can be measured directly, but the surface tension of a solid (or its surface energy) cannot be measured directly. In both cases, measuring the surface tension or surface energy is about understanding and controlling the surfaces of liquids and solids so they will form strong bonds when they interact with each other.
Surface tension is easily measured through the commonly used maximum bubble pressure method, a Wilhelmy balance, or a du Noüy ring tensiometer.
Surface Tension Can Be Controlled Dynamically
Surface tension is a critical variable in manufacturing, and available sensors make it relatively straightforward to do a good job of measuring and managing the surface tension of their products.
One of the reasons they have this level of control is because of the ease of measuring dynamic surface tension. They can collect data in real time without much difficulty and respond to surface tension changes with appropriate adjustments of formulation or temperature. This means there aren’t any surprises later on in the process due to uncontrolled surface tension. Adhesives, paints, and coatings are generally manufactured with automatic and tight control of their surface tensions.
However, that leaves open the other part of the interfacial bond equation. While the surface tension of the adhesive or coating is well controlled, how do you exercise similar control over the surface energy of the solid you must adhere to?
As stated previously, there are measurements we can make that are not direct measurements of surface energy but that directly correlate with surface energy. We can use our understanding of surface tension to quickly and precisely measure how a liquid reacts to a solid surface, and from this measurement, we can make a predictive inference about the surface energy level of that solid.
This sounds complicated, but it is really quite easy. And once we can easily make this measurement, then we can easily control the surface energy of solids and predict the strength and reliability of interfacial bonds in manufacturing processes.
What is the Relationship between the Surface Tension of a Liquid and the Surface Tension of a Solid?
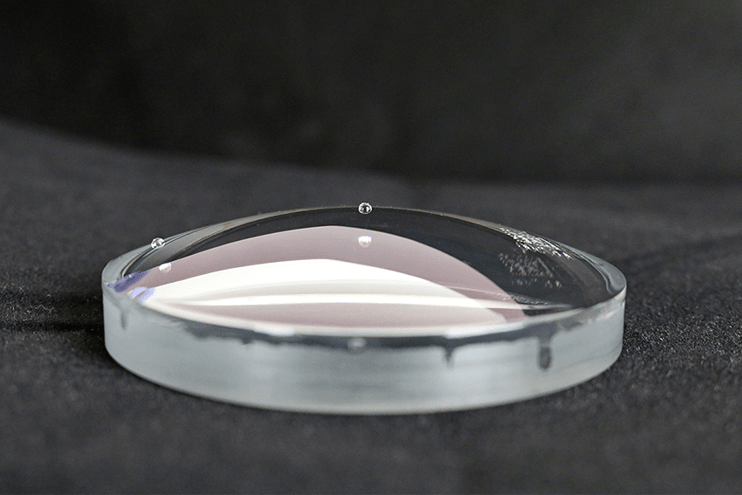
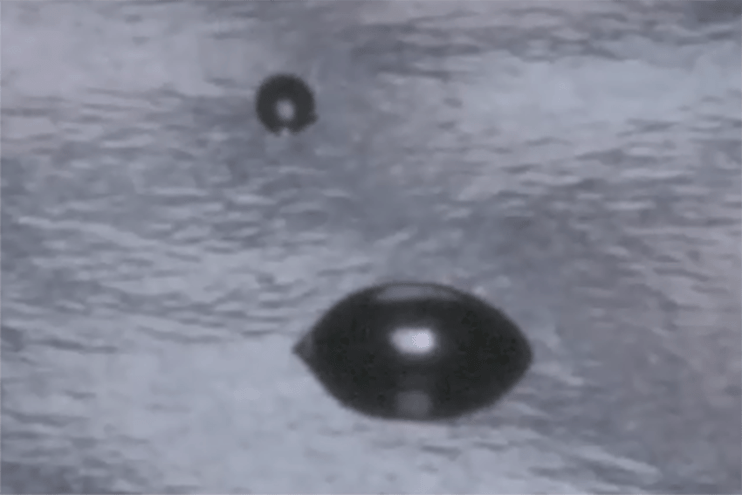
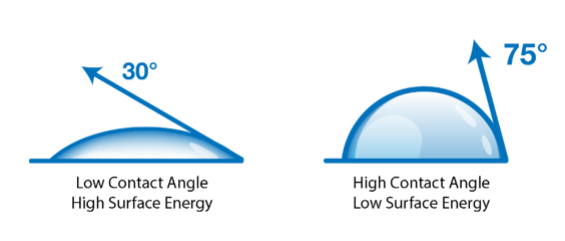
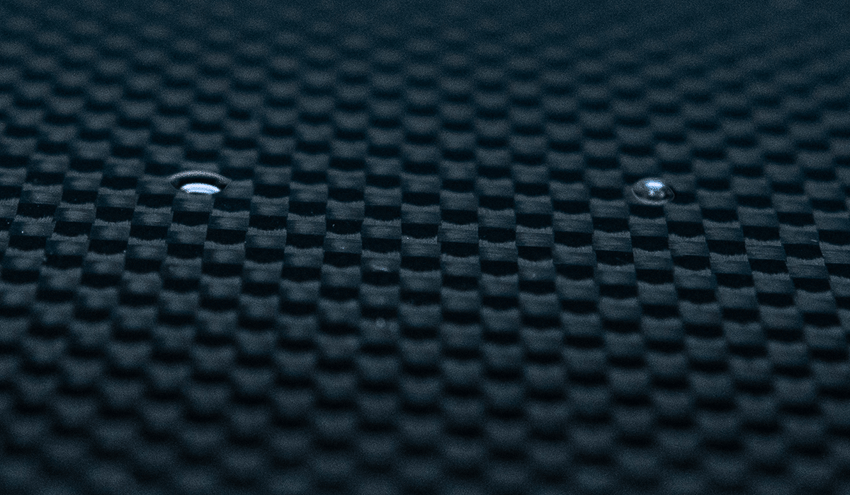
When a liquid comes into contact with a solid, it will either bead up or spread out. We see this all the time in the real world, with rainwater beading up on a waxed car or dishwater sliding off a clean plate in broadsheets. This interaction is determined by the interplay between the factors that determine the surface tension of the liquid and the factors that determine the surface energy of the solid.
If we know the surface tension of the liquid, then we can use that information to determine the surface energy of the solid. By measuring the behavior of a drop of liquid on a solid surface, we can use the correlation of that measurement with the surface energy of the solid to give us a level of control over processes that prepare, treat and clean surfaces for bonding that hasn’t been achieved in a production setting before.
For reliable and predictable adhesion, three things must be controlled:
- The composition and chemical characteristics of the adhesive, ink, paint, or coating
- The quality of the solid surface being bonded to
- The application and curing process of the adhesive, ink, paint, or coating.
Factors two and three are very well understood and controlled in manufacturing. The second factor, surface quality, is poorly controlled in most manufacturing operations, frequently because it is under-appreciated and tools for convenient measurement of surface properties are relatively new. As a result, variability and unpredictability in processes that depend on the adhesion of one material to another are left uncontrolled.
Optimize the power of next-gen connectivity with data & surface intelligence.
When it comes down to it, manufacturers can rely on the formulators of their adhesives and coatings to measure and control surface tension properly. What they must not do is leave the quality (i.e., cleanliness or treatment level) of their material surfaces to chance. The chemical attractions that bond substances together occur on a level that is invisible to the eye. So, just because a surface has undergone a series of process steps and appears clean does not mean it is ready to establish a strong, reliable bond. These properties have to be measured to ensure consistency.
To learn more about contact angle and how to leverage it in your operations, read the eBook "What is Contact Angle? Bridging the Gap: How Contact Angle Insights Drive Manufacturing & Supply Chain Innovations."