Automotive supply chains are complex. Nearly 78 million vehicles were manufactured in 2020. Each vehicle may have upwards of 30,000 individual parts. Automotive original equipment manufacturers (OEMs) need to manage billions of parts annually. If any of these parts fail, vehicle performance is impaired, brand perception is damaged, and safety issues may arise. OEMs need to maintain quality control over each part. This is a daunting task.
OEMs use Tier 1 suppliers to help manage this complexity. After qualifying a Tier 1 supplier, OEMs frequently rely on Tier 1 suppliers to provide parts that meet functional engineering requirements at the required production volumes and within the time frames. These Tier 1 suppliers may choose to manufacture these parts themselves, qualify Tier 2 suppliers to provide additional capacity, or create some type of hybrid model (with a combination of producing some parts internally and some parts externally). Mechanical fasteners are one type of part that may be manufactured by a Tier 2 supplier.
The Use of Anti-Corrosion Coatings on Mechanical Fasteners in the Automotive Industry
High volumes of mechanical fasteners are processed through the automotive supply chain. High-performance anti-corrosion coatings are an integral part of these mechanical fasteners. Anti-corrosion coatings provide rust protection, lubrication benefits, and protection from harsh environments. The success of these anti-corrosion coatings relies on chemically clean surfaces prior to coating application in order to achieve good adhesion and successful performance. Complex geometries, including threads and recesses, can make effective cleaning particularly challenging.
Given the extraordinarily high volume of mechanical fasteners used in the automotive industry, Tier 1 and Tier 2 suppliers have developed standardized production processes that are well-suited for the high-speed mass production of mechanical fasteners. Although these suppliers can quickly manufacture high volumes of products, historically, they have used cleaning processes that were not well-controlled. Production disruptions due to unplanned cleaning chemistry changeovers and inconsistent coating adhesion and corrosion performance are common. A lack of controlled, consistently reproducible cleaning processes results in the inconsistent quality of anti-corrosion coatings, which leads to increased scrap rates and, ultimately, to damaged brand perception.
The Cleaning Methods that Precede Application of Anti-Corrosion Coatings
The parts must be thoroughly cleaned to prepare them for the application of anti-corrosion coatings. This is typically achieved by cleaning the parts in a series of cleaning baths and rinsing baths. The initial cleaning steps focus on removing contaminants from the metal surface. The freshly cleaned metal parts are then submerged in a rinsing bath to remove any residues left by the cleaning agents and generate a surface ready to accept the anti-corrosion coating with maximum adhesion and performance.
What is the Maximum Cleaning Capacity of Your Chemical Bath?
The best cleaning results are achieved when the cleaning solutions and the rinsing baths are fresh. As the cleaning solutions and rinsing baths age, they become less effective due to contamination. (By analogy, consider the challenges of washing the dishes on a camping trip with only two bins of water for cleaning – one containing a detergent to clean the dishes and the second to rinse off the detergent residue. Although the first few dishes may be cleaned this way, the final dishes, rinsed in a second bin that has become increasingly contaminated with the residue of prior rinsed dishes, will be less clean than the first few washed dishes.)
“Bath loading” is the term used to describe the finite capacities of cleaning chemistries. Once a bath has reached this capacity, it can no longer trap additional contamination. At this point in the process, the bath is too full of soil to remediate additional contamination.
“Drag over” describes when cross-contamination occurs between baths. Specifically, when the cleaning solution clings to the surface of a part and is ‘dragged over’ to the next cleaning or rinsing step, the subsequent bath becomes contaminated.
Does Your Cleaning Chemistry Match Your Anticipated Soils?
Generally, a surface preparation process for anti-corrosion coating and plating must achieve two goals: first, remove any relevant soils, including particulate and chemical residues, from the part surface. The second goal is to create a pristine uniform oxide on the part, often through a combination of etching and de-smutting, which will allow the anti-corrosion coating or plate to form efficiently and adhere well to the part surface. Therefore, it is critical to ensure that the cleaning chemistry utilized is capable of removing the chemical residues present on the surface.
Rethink your adhesion manufacturing processes with Surface Intelligence.
The cleaning chemistry in each bath needs to be matched to the anticipated type of soil present on the mechanical fastener. Selecting a cleaning chemistry that matches the source of contamination is critical – if this matching is done incorrectly, the cleaning chemistries will not work.
Depending on your particular needs, a multistage cleaning and rinsing process may be designed that is more comprehensive than the two-stage cleaning and rinsing step described above. A multistage cleaning process might include three pairs of vats. In this scenario, each pair of vats is designed to remove specific contaminants. The first pair is designed to remove soil, followed by a rinse to remove detergent. The next pair will remove and then rinse the oxide scale and/or smut, and the final pair will clean the parts through some form of the etching process.
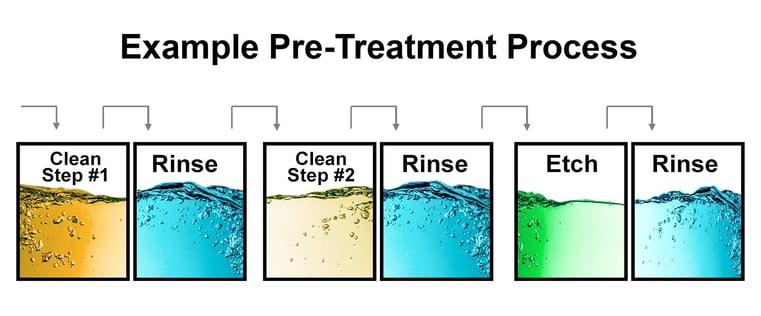
Checking the pH level of each bath in a multistage cleaning process is a legacy method of determining when it is necessary to add additional chemicals to a bath for cleaning purposes. However, adding additional chemicals to a bath does not eliminate contamination that is still present in the bath. Historically, chemical titration has been used to restore chemical baths to targeted pH levels.
Direct Measurements of Individual Parts Enables Better Control of the Cleaning Process
Seeking to control the pH level or chemical composition of a chemical bath is an indirect measurement of the effectiveness of the cleaning process. On the other hand, measuring the chemical cleanliness of individual parts is a direct measurement of the effectiveness of the cleaning process. Direct measurements of the surface cleanliness of the parts are superior to indirect measurements for maintaining the quality of the product. In the end, what is important for product quality is the cleanliness of the part surface, not the solution pH or cleaner concentration. Control of the cleaning process should be based on the quality of the part's surface.
Forward-thinking companies integrate quality control processes into their manufacturing that are based on direct measurement of important part characteristics, such as surface cleanliness, to minimize the opportunity for defects to occur.
Quantifying the Chemical Cleanliness of Parts Using Direct Measurements
Handheld or automated digital surface inspection devices that rapidly analyze contact angle measurements objectively determine whether the surface of individual parts has been properly cleaned prior to the application of anti-corrosion-resistant coatings.
Direct measurement provided by these digital surface inspection devices provides quantitative data that can be used as a digital thread that leverages historical data to trace the quality of part cleanliness. This digital thread also provides a common specification framework and common language that can help streamline communications between OEMs and their Tier 1 and Tier 2 suppliers. This digital thread provides proof to OEMs that their Tier 1 and Tier 2 suppliers are manufacturing parts to the required specifications.
Digital Surface Inspection Devices Empower Plating Shops to Stay Competitive within the OEM Supply Chain
Obtaining regular measurements of mechanical fasteners’ surface quality during the plating process will enable Tier 2 manufacturers to identify any point in the process when the cleaning baths reach contamination levels that require fresh chemistry. Establishing a testing framework on the surface quality of plated parts provides an objective, reliable feedback method. Using these devices to check part quality will provide a “trigger” to refresh the chemistry of the baths. This data will also validate to Tier 1 suppliers that the supplier responsible for the application of the corrosion-resistant coating is complying with production specifications.
Optimize the power of next-gen connectivity with data & surface intelligence.
As OEMs increase emphasis on ensuring that incoming materials are compliant with stated specifications, Tier 2 suppliers who can use a digital thread to document that their processes are in control and that they manufacture parts to designated specifications will have a competitive advantage in the marketplace relative to peer companies that cannot document process control to show that parts meet required specifications.
To learn more about how to effectively control surfaces for successful plating and coating, read the eBook "Metrics That Matter: Quantifying Cleaning Efficacy for Manufacturing Performance."