A major problem for manufacturers is the problem of sensitivity. There is a rampant lack of sensitivity to material surfaces for companies across every manufacturing industry. Granted, it’s not easy to test surface quality to predict the outcomes of common manufacturing processes like adhesive bonding, structural sealing, metal brazing, polymer adhesion, printing, painting, or coating. To be able to form a bond between two substances (i.e., glue and metal, plastic and plastic, paint to metal, ink to film, etc.), the materials need to have the proper chemistry at the top few molecular layers of their surfaces.
As a part of a complete quality assurance process, monitoring the chemical state of material surfaces is essential to ensuring the final product meets performance demands. But it’s something companies are struggling to do and do well.
The struggle comes from the fact already mentioned—that testing for contamination at the molecular level can be daunting and difficult to implement, especially on the production line where that information is most crucial.
Manufacturers need:
- An understanding of where their current tests might be insufficient
- A good alternative to those tests
- A trustworthy standard that offers a pathway to a solution for overcoming minute variables that throw giant wrenches into production processes
To illustrate the frustration many manufacturers face with molecular-level contamination causing enterprise-level disruption, we’ll take a look at a major packaging company that was having trouble with a heat sealing process and how they overcame it.
Rethink your adhesion manufacturing processes with Surface Intelligence.
What’s the Difference Between “Good” and “Bad” Material for Manufacturing?
The company was having intermittent problems heat sealing a polymer film used to package paper products. Essentially, the seal wasn’t reliably staying sealed, and this was causing real headaches for the customers that this company rightly doesn’t want to disappoint.
The company discerned that there was a film that failed performance tests and a film that held a strong seal. The trouble remained because the quality tests they were running were unable to tell the difference between the two. According to their findings, they seemed equivalent in every way. Yet, the seals kept failing.
They knew that the film looked identical, so they deduced that the problem must be something invisible to the eye on the surface. To try to find out if there was anything detrimental present on the surface, they used a very common surface analysis technique called dyne solutions.
Dyne inks are the most frequently encountered method of surface energy evaluation used on production lines or in manufacturing in general. The surface energy of a substance generally refers to how attractive it is to other substances: a high-energy surface is generally attractive to other substances; a low-energy surface is not.
Dyne inks consist of a series of liquids with a range of surface energies. The various surface tensions in the inks are created by mixing two or more mutually soluble liquids of different surface tensions in different proportions. Commercially available mixtures include ethanol and water or formamide and 2-ethoxyethanol. Usually, a blue or purple dye is added to the mixture to improve visibility on the surface.
In use, a thin film of the liquid mixture is spread on the surface to be tested; if the liquid film breaks up into droplets in less than two seconds, the test is repeated with a mixture of lower surface energy. The surface energy of the liquid mixture that will stay spread in a continuous film on the surface for at least two seconds is defined as being equal to the energy of the surface. This is just an approximation of the surface energy, however, and suffers from low accuracy and precision as well as poor reproducibility.
Furthermore, because dyne inks are solvents, they clean contaminants from the surface during measurement and therefore frequently do not detect them.
That was the problem this company was running into. The dyne pens showed no differences between a “good” film and a “bad” film. For the purposes of this article, we are just referring to the films as good or bad based on whether they sealed correctly or not.
Brighton Science took a look at what the dyne inks were doing to the surface and found that they cut through a contaminant found on the surface of the bad films, leaving the packaging company with a false sense of security. These false “all clear” signals from the dyne ink tests inevitably led the company to scramble to understand why they were disappointing their customers.
How to Detect Invisible Differences in Surfaces
When Brighton Science analyzed the good and bad films, the water contact angle was the first tool we used to baseline the differences between films.
Contact angle methods of measuring surface energy involve measuring how a drop of liquid placed on the surface spreads, usually by measuring the angle formed by the perimeter of the drop and the surface. A liquid drop that spreads a lot has a small contact angle; this means it is attracted to the surface more strongly than it is attracted to itself. A drop that spreads very little (‘beads up’) has a large contact angle: its molecules are being attracted more strongly to themselves than to the surface. The contact angle of the liquid on the surface changes with the chemical state of the surface in a very predictable and reproducible way.
We initially found higher water contact angles on the good film compared with lower contact angles on the bad film (Figure 1). This was unusual since, typically, a higher contact angle indicates a less-active surface that will not bond or seal correctly, but in this case, the lower contact angle was not bonding correctly.
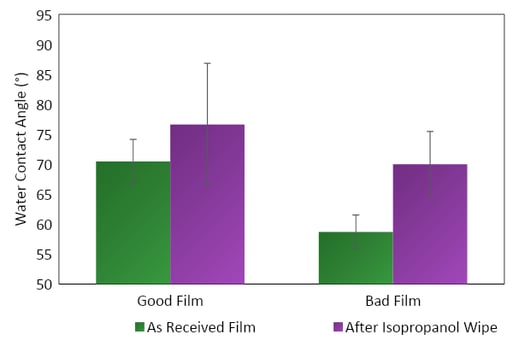
Figure 1. Water contact angle measurements on known good and known bad films before and after a wipe with isopropanol.
The contact angles of the films increased with the isopropanol wipe, indicating that whatever was causing the bad seal was soluble in the isopropanol. The films were brought to a similar state of energy after the wipe.
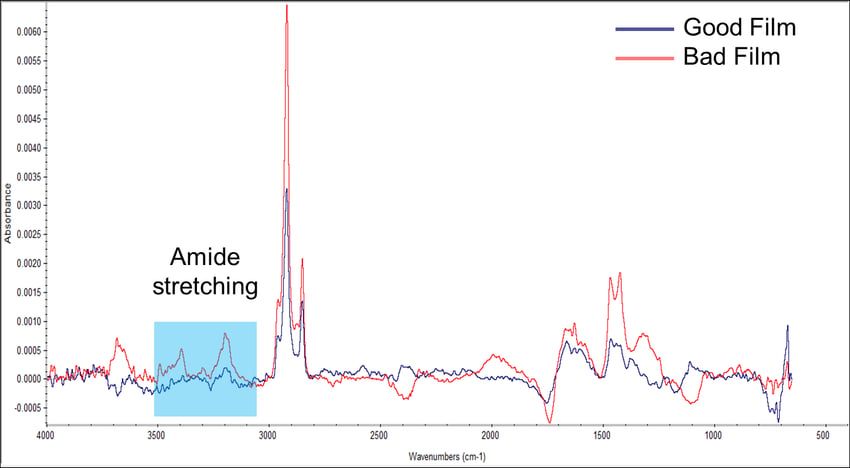
The films were then analyzed using infrared spectroscopy. Brighton Science was able to remove various substances from the films and identified that the bad film contained an amide group that the good film did not have (Figure 2). In the spectra, you can see the red line for the bad film has two much higher peaks in the blue box than the blue line for the good film. Activity within this range indicates the presence of this amide group.
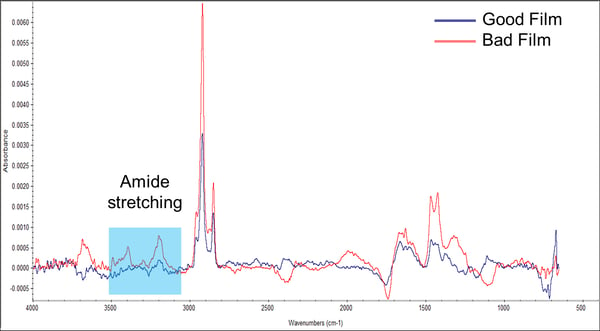
Figure 2. Infrared spectra of known good and known bad films.
Amide groups are generally hydrophilic (attractive to water), while the polymer film is hydrophobic (water-repellant). So, that answered the question of why the bad film had a lower contact angle. The water was wetting out because it was coming in contact with this hydrophilic amide group.
Upon more investigation, we found that the contaminating amide group was contained in the film additives. This blown film requires a lubricant, also called slip, in the formulation to reduce friction on the film as it passes through rollers and during the cooling process. These processing aides are known to reduce friction but can cause challenges for adhesion and heat sealing when present in excess. The slip molecule, a fatty acid amide, contains an amide group, which was identified through chemical analysis. This was present on the bad films, not the good films.
The typical state of the good film before treatment was that of a fairly low-energy surface with contact angles in the 70s. Corona treatment is required to bring those contact angles lower and increase the surface energy so that the film will seal properly. When the film is being treated, it needs to slide smoothly and effortlessly over the rollers that send it through the machinery.
Slip reduces the coefficient of friction of the film, so it makes the journey over the rollers freely and prevents any gunk from building up on the rollers that can stick to rollers. Hot polymers tend to stick to metal rollers used during the blown film process. If the slip was not present, then the rollers could require additional cleaning, they could transfer unwanted substances onto the film, and if there are almost imperceptible catches in the film on the rollers, then there could be tiny creases in the film that won’t get treated and lead to similar sealing failures that this packaging company was seeing. So, slip serves a very important purpose, but excess can be detrimental to sealing.
Where Do Contaminants Come From?
There are several reasons why excess slip could appear on the surface of a film. There may have been too much slip added to the film formulation of this packaging. There could have been leftover slip on the rollers from a previous batch of a different kind of film. Temperature changes in the production facility could have an impact on the slip molecules’ behavior.
Optimize the power of next-gen connectivity with data & surface intelligence.
A hot day can cause more slip to migrate to the surface because when polymers heat up, the molecules become mobile, and substances that are supposed to stay in the bulk of the material can make their way outward. Similarly, yet not as common, temperatures that are too cold can cause what’s called phase separation to occur between the slip agent and the rest of the polymer. Think of it when you put homemade soup in the fridge, and the fat within the bulk of the soup solidifies at low temperatures and makes its way to the top of the soup. That happens because the fat has a phase separate from the water. When the soup is heated back up for eating, the fat molecules disperse back into the rest of the soup.
No matter the cause of the excess slip, the film manufacturer now has a way to measure and monitor it because it can be found using a water contact angle.
Slip is soluble in alcohol and many other solvents, including those found in dyne pens (Figure 1). In this case, the dyne pen ink could not indicate the presence of slip because it dissolved the ink. However, slip, while somewhat hydrophilic, is not very soluble in water, so water could react to its presence without dissolving it. Using water as the probe fluid (as scientists refer to it), the test was able to be sensitive to what was actually happening on the surface of the film.
We were able to help this film manufacturer use contact angle measurements to monitor excess slip in their process. When you inspect surface quality with this method at all the places where contaminants, like slip, can cause problems or where they can be dealt with, then you can tackle the variables that are causing friction (of a sort, in this case, the slip was reducing friction but increasing emotional and economic distress). We call these problems areas Critical Control Points.
Measuring at your Critical Control Points, such as directly on the rollers, allows manufacturers to discover leftover slip residue between batches. Measuring before and after treatment lets you know when to change corona treatment levels to change slip migration. Measuring in various temperatures can determine when a hot day of production is too hot for slip migration. Measuring the surface quality of incoming materials gives you the opportunity to catch variations so you can add a cleaning step for “bad” materials if necessary or hold your suppliers accountable for inconsistent parts.
Using contact angle measurements to investigate potential causes for excess slip or the presence of any contaminant detrimental to building reliable products can be the difference between having satisfied customers and having frustrated clientele.
To learn more about implementing quality control methods that secure your manufacturing process from start to finish, download our eBook, " What is Dyne Testing?"